The Claus and Modified-Claus Reaction
The Claus process was first patented in 1883 by the German chemist Carl Friedrich Claus. His discovery, the Claus Reaction, is the basis for what has become the industry standard for sulfur recovery.
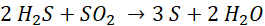
This basic chemistry occurs in both the Thermal Reactor (thermal process) and across multiple catalytic reactors (catalytic process) of a traditional Sulfur Recovery Unit.
The Modified-Claus process expands on this reaction to directly process Hydrogen Sulfide (H2S) bearing Acid Gas Streams by oxidizing one-third of the incoming H2S to produce the SO2 necessary to initiate the Claus Reaction. The combined multi-step Modified-Claus reactions include:
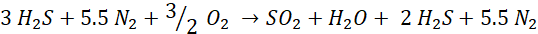
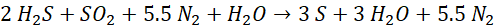
The Nitrogen (N2) shown above is not part of the Modified-Claus reaction but is shown here as part of the Combustion Air normally used in Sulfur Recovery Units. This Nitrogen impacts the throughput capacity of an SRU. See Oxygen Enrichment and Debottlenecking for more information.
In a real-world process, there are many side reactions that occur, including the combustion of hydrocarbons present in Acid Gas, the oxidation and dissociation of ammonia, and the formation and destruction of Carbonyl Sulfide (COS), Carbon Disulfide (CS2), and Oxides of Nitrogen (NOx).

